Over 4.5 million people in the UK are known to have diabetes and an estimated 1.2 million people are thought to have undiagnosed diabetes. Most people have type 2 diabetes which can be managed in primary care until complications develop (McCombie et al, 2017; Diabetes UK, 2023). Effective management in primary care can enable people with diabetes to manage their condition well and reduce the rate of complications. This article, part of an occasional series on post-pandemic diabetes care, discusses the role of GLP-1 receptor agonists (GLP-1) in managing type 2 diabetes.
A growing problem
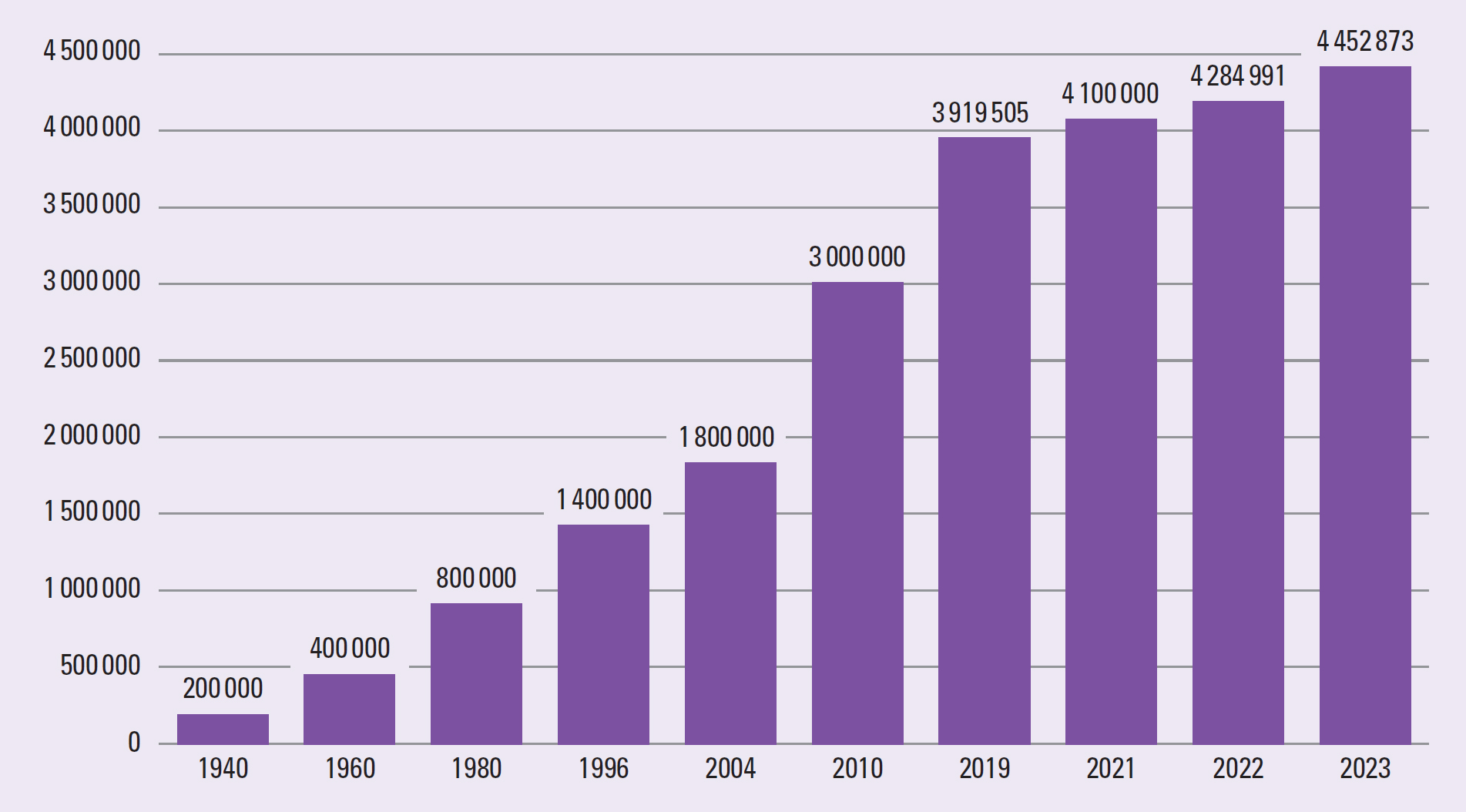
The World Health Organization (2023) defines diabetes as a chronic, metabolic disease characterised by elevated levels of blood glucose (or blood sugar), which over time can lead to serious damage to the heart, blood vessels, eyes, kidneys and nerves. Since 1940, the number of people in the UK with a diabetes diagnosis has risen from 200000 to around 4.5 million and an estimated 1.2 million people with diabetes remain undiagnosed (Diabetes UK, 2023). Figure 1 illustrates the rising numbers of people diagnosed with diabetes.
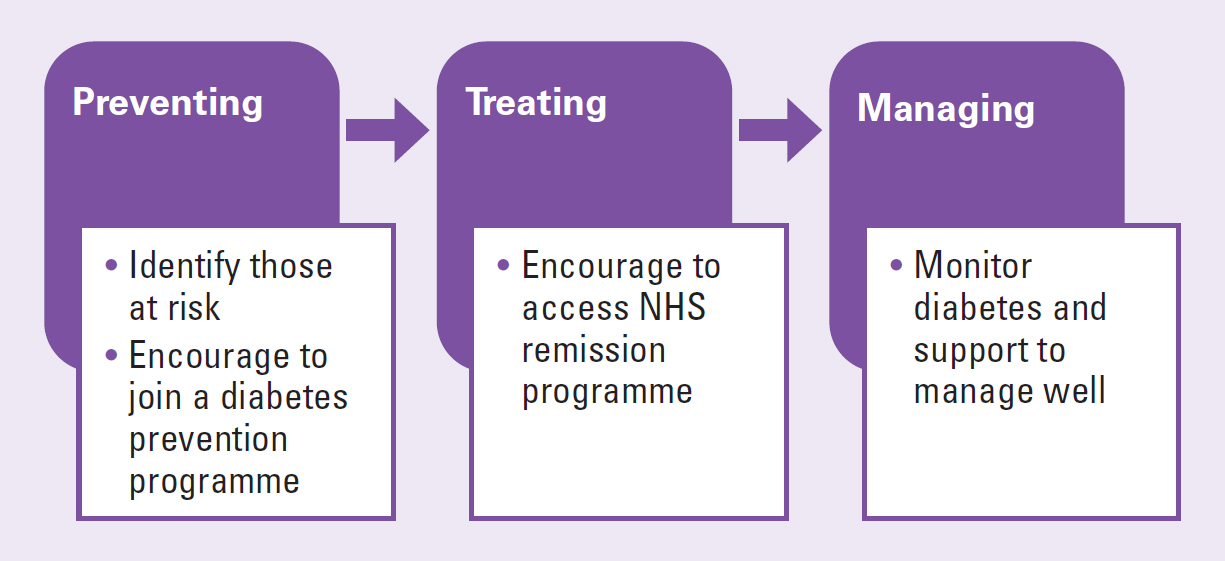
There are three components to diabetes care. These are prevention, treatment and management (Figure 2).
Prevention and treatment of type 2 diabetes
Currently 7% of adults in England have diabetes and 12% are thought to be at risk of developing type 2 diabetes (ONS, 2024). The NHS has set up diabetes prevention programmes across the UK, which involve intensive behavioural interventions and support people with weight loss, diet and physical activity. The programmes aim to enable people to make simple lifestyle changes to reduce the risk of developing diabetes. The programmes are open to people who are not pregnant, are aged between 18–79 years, do not have diabetes currently and have received a score of 16 or over when using the ‘know your risk’ tool. Links to the assessment tool and the programmes are in the resources section.
The way type 2 diabetes is treated has changed dramatically in recent years. Research shows that weight loss can lead to a remission of type 2 diabetes. The DiRECT trial found that 86% of people with type 2 diabetes who had been diagnosed in the previous 6 years could achieve remission if they lost weight rapidly. In overweight and obese people, the greater the weight loss the higher the level of remission. A loss of 15kg led to 86% remission and a weight loss of 10kg led to a 46% remission (Lean et al, 2018).
NHS England has introduced a programme to enable people with type 2 diabetes to lose weight and put their diabetes into remission (NHS England, 2022). The NHS Type 2 Diabetes Path to Remission Programme (formerly known as the NHS Low Calorie Diet Programme) is being rolled out across England. It provides a meal replacement programme of soups and shakes for three months, providing around 900 calories daily. The programme also provides ongoing support from clinicians and coaches to enable people to maintain healthy lifestyles (NHS England, 2022). Scotland and Wales have similar programmes (Counterweight, 2022; NHS Lanarkshire, 2024). Pregnant women are not eligible for the weight loss programmes. Table 1 provides details of eligibility.
| Criteria | Details |
|---|---|
| Age | 18–65 |
| Diagnosed | Within the last 6 years |
| BMI | BMI over 27 kg/m2 if from White ethnic groups BMI over 25 kg/m2 if from Black, Asian and other ethnic groups |
Management of type 2 diabetes
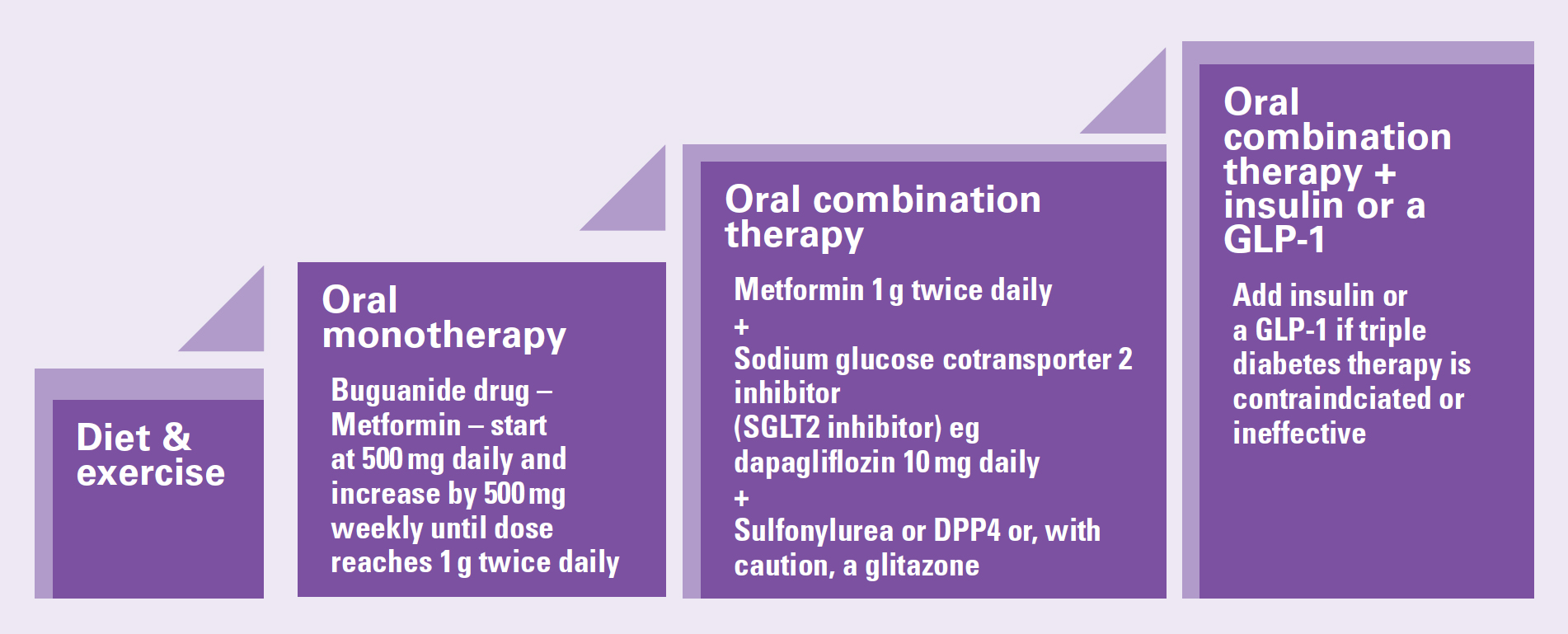
Managing type 2 diabetes aims to enable a person to change his or her lifestyle to improve diet and exercise more, assisting weight loss and controlling blood glucose levels. People who have good glycaemic control have a reduced risk of complications from diabetes (NICE, 2022a). A step-wise approach to treatment is recommended (NICE, 2022a) (Figure 3, Box 1).
Case study
Charles is a 49-year-old father of four. He is originally from Somalia and works as a bus driver. Charles’ wife died of breast cancer three years ago and since then Charles been both a father and a mother to his children. He has learnt to cook and every evening provides his children with a home cooked meal. Afterwards he supervises their homework. Charles has gained a lot of weight since his wife died and he isn't active during the day. His BMI is 31. He has developed type 2 diabetes that this is not well controlled despite triple therapy. In the past, Charles would have been put on insulin and would not have been able to drive a bus, as per DVLA rules. This would have had a major effect on his and his children's lives, because being required to do non-driving duties would have significantly reduced his income. Charles was prescribed exenatide to be taken once weekly and joined the NHS remission programme. Six months later Charles’ BMI is 26, he has lost 16 kg and learnt how to cook food with less calories. He is now on two medications, Metformin 2 g modified release once daily and dapagliflozin 10 mg once a day. Charles no longer requires Exenatide to treat his diabetes, feels much better, and continues to drive buses.
Medication
A number of different classes of medication can be used to treat type 2 diabetes. These are biguanides, SGLT2 inhibitors, DPP-4 inhibitors, glitazones, sulphonylureas, GLP-1 and insulin (Table 2). Metformin is used as a firstline medication and a sodium glucose cotransporter-2 (SGLT2) inhibitor is added when it is confirmed that metformin is tolerated. If dual therapy is not effective, a third antidiabetic agent is added. This could include sulfonylureas, DPP-4 inhibitors known as gliptins and pioglitazone, which is the only glitazone licensed for use in the UK. When triple therapy is not effective, insulin or a GLP-1 receptor agonist is added to the treatment regime (NICE, 2022; Nazarko, 2023).
| Name | Mode of action | Dosage | Comments |
|---|---|---|---|
| Dulaglutide: As monotherapy if metformin inappropriate | GLP-1 | 0.75 mg once weekly | May be given as monotherapy, first line treatment, metformin, inappropriate (NICE, 2024c) |
| Dulaglutide: As part of combined therapy | GLP-1 | 1.5 mg once weekly; increased if necessary to 3 mg once weekly after at least 4 weeks, then increased if necessary to 4.5 mg once weekly after another 4 weeks A starting does of 0.75 mg once weekly may be considered for potentially vulnerable patients. | Maximum dose 4.5mg weekly (NICE, 2024c) |
| Exenatide | GLP-1 | 2 mg once weekly | Give once a week on the same day each week (at any time, with or without meals). The day of weekly administration can be changed in necessary, as long as the next dose is administered at least 24 hours later (NICE, 2024d) |
| Liraglutide: | GLP-1 | 0.6 mg once daily, increased after at least 1 week to 1.2 mg once daily. This can be further increased, if necessary, after an interval of at least 1 week to a maximum dose of 1.8 mg once daily | (NICE, 2024e) |
| Lixisenatide: | GLP-1 | 10 micrograms once daily for 14 days, increased to 20 micrograms once daily thereafter. | To be taken within 1 hour before the first meal of the day or the evening meal (NICE, 2024f) |
| Semaglutide | GLP-1 | 0.25 mg once weekly for 4 weeks, then 0.5 mg once weekly for at least 4 weeks, then increased to 1 mg once weekly if needed | (NICE, 2024g) |
| Tirzepatide | GIP analogue activates both the GLP-1 and GIP receptors | 2.5 mg once weekly for 4 weeks, then 5 mg once weekly for at least 4 weeks, then increased if necessary up to 15 mg once weekly, with doses increased in steps of 2.5 mg intervals of at least 4 weeks | (NICE, 2024h) |
GLP-1 agonists
There are six GLP-1 agonists: exenatide, liraglutide, lixisenatide, dulaglutide, semaglutide and tirzepatide (NICE, 2024a). All GLP-1 agonists are modified release and are administered weekly by subcutaneous injection. GLP-1 medication is given by injection (Table 2) because gastric acid and proteases would affect absorption if given orally.
A tablet form of semaglutide is now available. It is given daily with a small amount of water after an overnight fast, following which no food or medication should be taken for 30 minutes. The tablet is specially formulated to improve absorption. However, bioavailability is low: a 14 mg tablet is equivalent to a 0.5 mg weekly injection (NICE, 2024f). Exenatide immediate release, a GLP-1, that was injected twice a day, was discontinued in March 2024. Prescribers were directed to switch people treated with exenatide immediate release to oral semaglutide (Department of Health and Social Care, 2024).
How GLP-1s work
Healthy adults maintain stable blood glucose levels (glucose homeostasis) because of the interplay of multiple hormones including insulin, amylin, glucagon and incretin hormones. The incretin hormones, GLP-1 and glucose-dependent insulinotropic polypeptide (GIP) are released on eating and lead to 50–70% of insulin secretion after a meal. Incretins are less effective in people with type 2 diabetes (Gilbert and Pratley, 2020). When food enters the gut, the hormone GLP-1 is secreted by enteroendocrine L cells of the small intestine (Kasina and Baradhi, 2024). This stimulates insulin secretion, suppresses glucagon secretion and slows gastric emptying. These actions lead to a reduction in blood glucose levels (Collins and Costello, 2024). Human GLP-1 is not suitable for therapeutic use as it has a half-life of less than 2 minutes (Kasina and Baradhi, 2023). GLP-1 receptor agonists have a longer action and they bind to and activate GLP-1 receptors in the pancreas. Like human GLP-1, they lower glucose levels by stimulating insulin secretion, suppressing glucagon secretion, delaying gastric emptying and inducing satiety (Nauck and Meier, 2017).
GIP is released from K-cells in the duodenum and proximal gut in response to food. It enhances insulin secretion from pancreatic beta cells but it does not appear to suppress glucagon secretion in the same way as a GLP-1 (Gilbert and Pratley, 2020). Human GIP is not suitable for therapeutic use as it has a half-life of less than 7 minutes (Gupta and Kalra, 2011). The medicine tirzepatide is a GIP analogue that activates both the GLP-1 and GIP receptors (Ma et al, 2023; Collins and Costello, 2024).
Indications
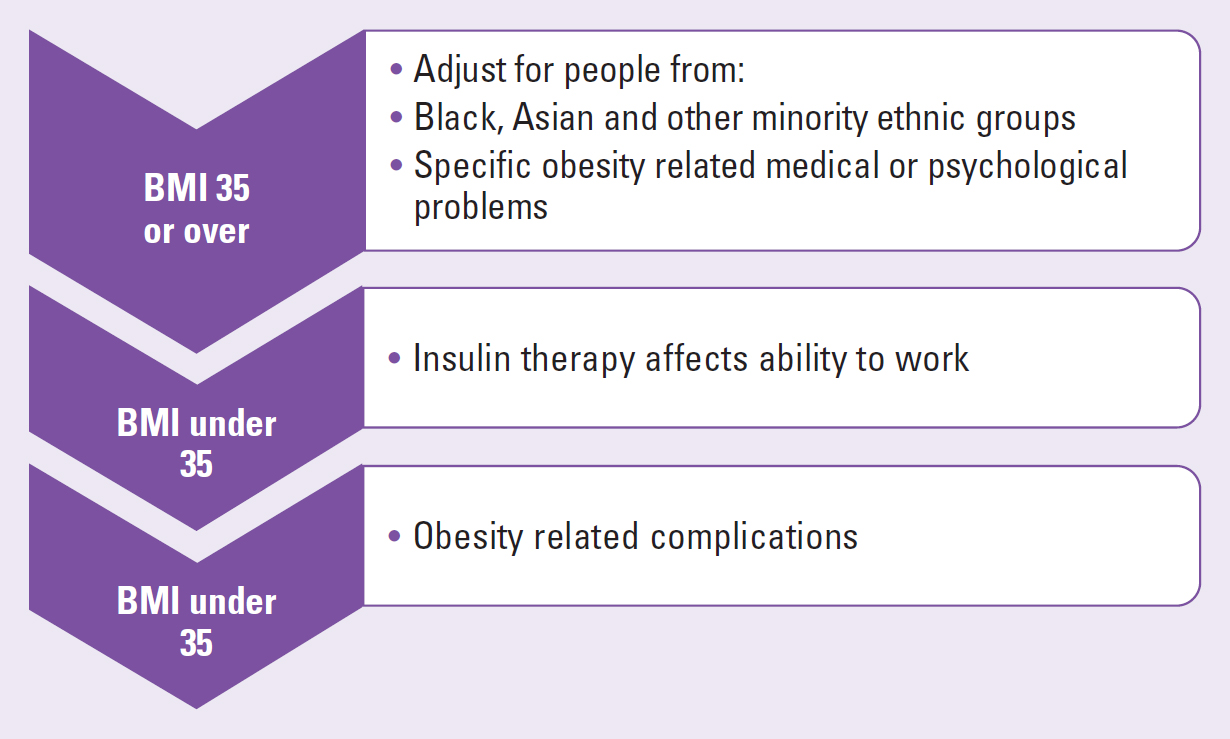
When triple therapy is not effective, insulin or a GLP-1 receptor agonist may be added to the treatment regime (NICE, 2022; Nazarko, 2023). Figure 4 shows the indications for GLP-1.
Adverse effects of GLP-1 medicines
Gastrointestinal side effects are most common and include nausea, vomiting and diarrhoea. This can lead to dehydration and acute kidney injury. Dizziness, mild tachycardia, infections, headaches and dyspepsia may also occur. If nausea is present, doses should be increased slowly and cautiously. Itching and erythema commonly occur at injection sites (Collins and Costello, 2024).
Research suggests that semaglutide has the greatest risk of nausea, diarrhoea, vomiting, constipation and pancreatitis, while liraglutide has the greatest risk of upper abdominal pain (Liu et al, 2022). Less common side effects include an increased incidence of gallbladder or biliary disorders, especially with higher dosage and prolonged use (He et al, 2022). Uncommon side effects include pancreatitis, alopecia, renal impairment and tachycardia (Filippatos et al, 2014). Semaglutide and tirzepatide should be used with caution in people with retinopathy (NICE, 2024a).
GLP-1s generally do not cause hypoglycaemia alone. However, they can contribute to hypoglycaemia if used in combination with a sulphonylurea and insulin (Filippatos et al, 2014). It can be dangerous to stop either a sulphonylurea or insulin, or adjust too quickly. The MHRA (2019) advises careful blood glucose monitoring and stepwise adjustment of insulin because of previous reports of life-threatening cases of diabetic ketoacidosis in association with exenatide, liraglutide and dulaglutide, particularly after discontinuation or reduction of concomitant insulin. It also recommends advising patients of the risk factors for, and signs and symptoms of, diabetic ketoacidosis, as well as advising them to seek immediate medical assistance if these develop. Table 3 outlines side effects of GLP-1 medicines.
| Drug | Frequency of side effects | ||
|---|---|---|---|
| Very common (>1 in 10) or Common (between 1 in 10 and 1 in 100) | Uncommon (between 1 in 100 and 1 in 1000) | Rare (between 1 in 1000 and 1 in 10 000) or Very rares (<1 in 10 000) | |
| Exenatide prolonged release (Bydureon®) | Appetite decreased; asthenia; constipation; diarrhoea; dizziness; gastrointestinal discomfort; gastrointestinal disorders; headache; nausea; skin reactions; vomiting | Alopecia; burping; drowsiness; hyperhidrosis; renal impairment; taste altered | Severe pancreatitis (sometimes fatal), including haemorrhagic or necrotising pancreatitis, has been reported rarely; discontinue permanently if diagnosed. |
| Lixisenatide (Lyxumia®) | Back pain; cystitis; diarrhoea; dizziness; drowsiness; dyspepsia; headache; increased risk of infection; nausea; vomiting | Urticaria | |
| Liraglutide (Victoza®) | Asthenia; burping; constipation; diarrhoea; dizziness; dry mouth; gallbladder disorders; gastrointestinal discomfort | Malaise; pancreatitis; tachycardia; urticaria | Renal impairment |
| Dulaglutide (Trulicity®) | Appetite decreased; atrioventricular block; burping; constipation; diarrhoea; fatigue; gastrointestinal discomfort; gastrointestinal disorders; hypoglycemia; nausea; sinus tachycardia; vomiting | Anaphylactic reaction; angioedema; pancreatitis acute (discontinue) | |
| Semaglutide (Ozempic®) | Appetite decreased; urping; cholelithiasis; diarrhoea; dizziness; |
Taste altered | |
Cautions and contraindications
GLP-1 medicines are contraindicated in people with a history of medullary thyroid cancer or multiple endocrine neoplasia (MEN) type 2. It is also contraindicated in type 1 diabetes, pregnant or breast-feeding women, people with a history of pancreatitis, severe gastrointestinal disease (eg inflammatory bowel disease) and gastroparesis. They may be contraindicated or used with caution in renal disease depending on the level of renal impairment. They should be used with caution in people with high risk of pancreatitis, for example those with gallstones, alcohol excess, hypertriglyceridemia (NICE, 2024a).
Discussion
GLP-1 medication is relatively new and the long-term effects of such medication is not yet known. It is not clear how long GLP-1 medication can be taken for and professional opinions vary. Some professionals feel that a person requires lifelong treatment and that the person will regain lost weight if the medication is stopped. Research indicates that weight loss varies between individuals, but is typically around 1.5 kg to 6 kg. Weight loss tends to plateau at around 6 months (Nauck and Meier, 2019).
GLP-1 medication is increasingly being initiated in primary care. Some Integrated Care Boards are using a traffic light system and shared care protocols. In such systems, most GLP-1 medication is rated green and can be initiated and managed in primary care. Some medicines such as semaglutide are rated amber and are a part of shared care agreements. Some medicine combinations such as insulin and a GLP-1 can only be given with specialist care advice and ongoing support from a consultant-led multidisciplinary team.
There is an interplay between obesity, diabetes, chronic kidney disease and cardiovascular disease (Kumar and Blaha, 2024). Studies indicate that the benefits of GLP-1 medications extend beyond weight loss and can improve outcomes for people with renal and cardiovascular disease (Marassi and Fadini, 2023)
Conclusion
The increase in type 2 diabetes is a major concern. It is a preventable pandemic that claimed the lives of 6.7 million people in 2021 (International Diabetes Federation, 2021). To reduce complications of poorly managed diabetes, the management of diabetes can no longer be restricted to endocrinologists and diabetes specialist nurses. All clinicians need to work together to support those at risk of type 2 diabetes to prevent, treat and manage the condition. This will have a major impact on the person's health and wellbeing.


