The lymphatic system acts as a mechanism to maintain fluid balance, immune response and nutritional absorption (Mortimer and Rockson, 2014), and dysfunction of this system leads to oedema. It has been estimated that between 3:99 and 28.76 per 1000 people are affected by chronic oedema, with lipoedema affecting 1:72 000 as a long-term condition (Moffatt et al, 2016; Wounds UK, 2017). Lipoedema is characterised by increased fat disposition within the subcutaneous layer and can lead to the development of excessive interstitial fluid in the dermal layer, which is the main characteristic of chronic oedema (National Lymphoedema Partnership, 2015; Wounds UK, 2017). Compression therapy is considered to be one of the mainstays in the management of chronic oedema and lipoedema. The application of compression therapy in the form of bandages, hosiery or adjustable compression devices (ACDs) is often used to manage lymphatic failure (International Lymphoedema Framework (ILF), 2012). This is usually alongside the modalities that enhance patient outcomes during the long-term management of the condition, such as simple lymphatic drainage (ILF, 2006).
| Manufacturer | Product name | Compression levels |
|---|---|---|
| BSN Medical | Jobst FarrowWrap lite, strong, classic | 20–30 mmHg/30–40 mmHg |
| Haddenham | EasyWrap light, strong | 20–30 mmHg/30–40 mmHg |
| Juzo | Compression Wrap | 20–50 mmHg |
| L&R | ReadyWrap | Up to 40 mmHg |
| Medi | Juxtacures/Juxtalite | 20–50 mmHg |
| Sigvaris | Compreflex, lite, CompriFit | 20–50 mmHg |
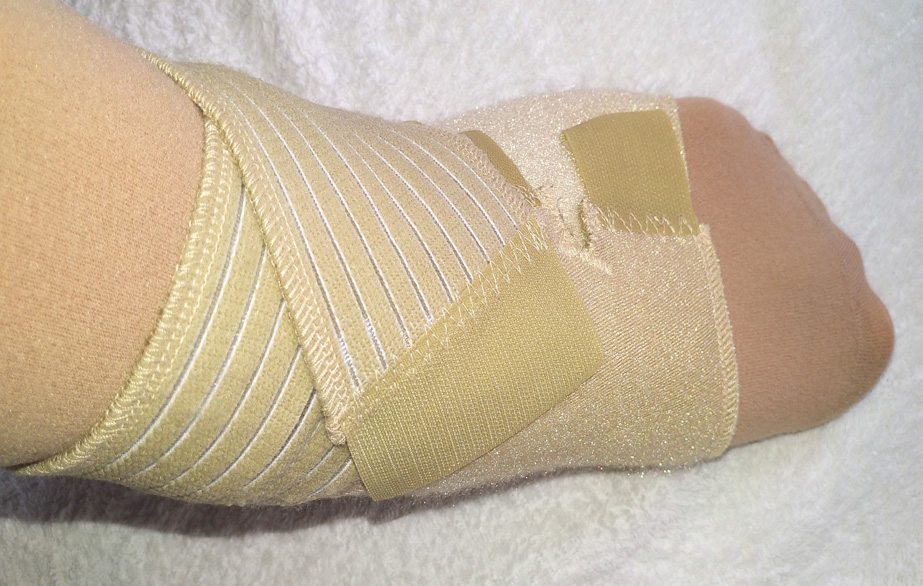
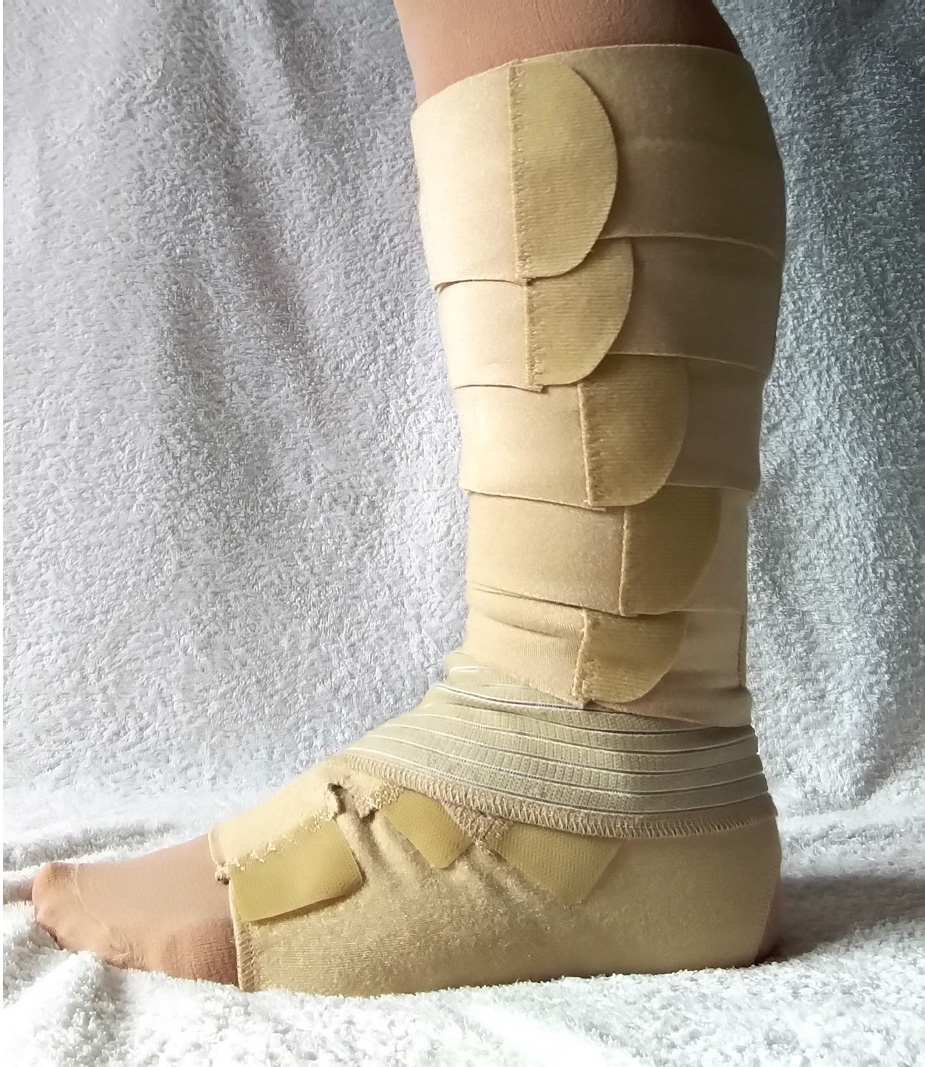
Adjustable compression devices
The advent of ACDs has presented new avenues in the management of chronic oedema, and these devices have gained popularity, with a number of companies manufacturing multiple versions (Figures 1 and 2; Table 1). Selection of the appropriate device for compression therapy requires consideration of the patient's needs, for care to be personalised to their situation, choice, pathophysiology and the psychosocial effects that they experience (NHS England, 2018a). Research shows that the rate of patient activation, whereby patients engage in various activities to manage their long-term condition, is between 60 to 75% (NHS England, 2018b). This means that between 25 to 40% of patients with a long-term condition are not engaging in activities to manage their condition due to a variety of reasons (NHS England, 2018b). Studies have also suggested that up to 50% of patients receiving compression therapy in the form of multi-layer lymphoedema bandages (MLLB) are non-adherent to treatment (Miller et al, 2011). However, it has been suggested that patients' knowledge as to why compression therapy is being used for their condition is often limited (Thomas, 2017). This can lead to some patients being dependent on the healthcare professionals instead of being empowered to lead in their own care (Balcombe et al, 2017).
The aim of compression therapy is multifaceted, and this treatment affects the patient's physiology to bring about several vital changes: (1) reduced capillary filtrate, (2) reduced fluid accumulation, (3) reduced presence of inflammatory mediators and (4) enhanced venous and lymphatic return/capacity (Rooney et al, 2018; Williams, 2018). Research by Partsch (2014) suggested compression levels should be considered against the interface pressure and stiffness of compression therapy garments, due to their effects on enhancing muscle contraction, and the intermittent compression of the veins/lymphatics. Stiffness is ascertained via the static sti?ness index (SSI) and how this alters the pressure from resting to standing in terms of narrowing or occluding of the venous lumen (Partsch et al, 2016). Partsch's (2014) study suggested that some ACDs may achieve a medium to high SSI, which is almost comparable to that achieved by short-stretch bandaging (SSB) systems (standing pressures of around 50 mmHg). The potential for ACDs to have a similar SSI as SSBs has prompted substantial research in this area of therapy.
Damstra and Partsch's (2013) prospective randomised study compared ACD to MLLB, and their findings showed that greater limb volume reductions were obtained with ACDs, and that these devices were better at sustaining the appropriate compression levels after application. However, the study was limited to a 24-hour period of application, rather than the 2–3 weeks that patients usually need compression therapy for (ILF, 2006). Mosti et al's (2015) study went further and considered the benefits of ACDs, including comfort, compared to bandaging with patients diagnosed with a venous leg ulcer. Groups were randomised to receive compression therapy with either SSB or an ACD, which lasted 1 week. The results indicated that limb volume reductions were higher in the ACD group, as were the reported comfort levels, due to the ease of reapplication/readjustment, aesthetic benefits and the ability to wear shoes. However, the study used an alternative method for measuring the limb volume, which is considered to be less accurate compared to the water displacement used in Damstra and Partsch's (2013) study. This was also one of the few studies to report on the limitations of ACD, with difficulties in application experienced by patients who are overweight, inflexible or have severe shape distortion.
A recent review by Williams (2018) examined the evidence surrounding ACDs and their application for patients with lymphoedema. This review found evidence that the use of ACDs aided in reducing oedema and the healing of leg ulcers. However, because of variations in the designs of studies included in the review, the generalisability of the evidence is quite limited. In one case study by Rooney et al (2018), MLLB was combined with an ACD, and the patient had to complete MLLB (with SSB), undertake 20 minutes of exercises, reapplying a third layer of SSB and undertake a further 20 minutes of exercise. After this was completed, the patient was fitted with an ACD to be worn for 24 hours and tightened every 2 hours over a 5-day period, which resulted in fluid reduction of 667 ml in that patient. Based on this review, the application of ACD has the potential to replace or augment MLLB in those patients who do not have severe distortion and who are able to self-care alone or with the support of others (carers). The use of ACDs also allows other healthcare professionals not trained in MLLB to administer compression therapy, thus increasing the number of people who could support patients with their treatment, whether it is for chronic oedema or venous leg ulcers.
Costs
During the selection of an ACD, expert opinion, the best available evidence and patient choice must be considered (Thomas, 2017). An assessment needs to identify the underlying pathophysiology to determine cause and any other factors that may affect patient outcomes, such as medication and other long-term conditions (ILF, 2006). The suitability of compression therapy when assessing for arterial disease can be challenging in patients with chronic oedema/lipoedema, which has led the British Lymphology Society (2018) to develop a position statement aiming to support the decision-making process in the application of compression therapy in the absence of ankle brachial pressure index findings, or if the reliability of these findings is questionable. If selected, the initial cost of an ACD can lead to a potential saving of £881 in bandaging costs, which is indirectly increased by the associated saving of clinical staff time, to up to £3174 over a 6-month period (National Institute for Health and Care Excellence, 2015). These figures are based on treatment of venous leg ulcers, but could be extrapolated to treatment of chronic oedema/lipoedema.
| Easy application |
| Adjustable by the patient/carer |
| Aesthetically acceptable |
| Colour variations |
| Upper/lower limb options |
| Mimics short-stretch bandaging (in terms of the static stiffness index) |
| Variable compression levels available |
| Can be worn with shoes |
Conclusion
In conclusion, the use of ACDs can be appropriate and effective in the management of chronic oedema/ lipoedema, given the reported ease of application for both patients and carers. However, use of ACDs for compression therapy must be based on a holistic assessment that attempts to understand all aspects of the patient's health, in order to ensure that the management of their condition is personalised to the exact point within their care journey. Both parties must be willing to engage with each other, for information and decisions to be shared equally. ACDs can allow healthcare professionals to better support self-care and offer potentially improved care if suitable following an assessment. This is especially relevant given that a high percentage of patients do not adhere to treatment (MLLB) or show poor activation. ACDs might help improve patient compliance with treatment. BJCN

