The number of COVID-19-related deaths surpassed 1 million at the time of writing (World Health Organization (WHO), 2020). Together with pre-existing respiratory illness, cardiac disease and its associated risk factors are significant risk factors linked to COVID-19-related mortality (Shoar et al, 2020). The disease caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) was first recognised as a respiratory infection. However, a better understanding of the manner in which the virus enters and affects the body suggests that its effects are more widespread and significantly influence the cardiovascular system directly and indirectly (Gavriatopoulou et al, 2020; Goha et al, 2020; Liu et al, 2020). This paper will examine the pathogenesis of COVID-19 and relate this to patients with heart failure. This examination will then be used as to identify important aspects of management relevant to primary care.
The link between COVID-19 and heart failure, or more specifically, heart disease is multifactorial. COVID-19 may have a direct or indirect effect on the cardiovascular system (Ambrosy et al, 2020; Babapoor-Farrokhran et al, 2020a; 2020b; Bader et al, 2020). The presence of chronic heart failure in COVID-19 patients is associated with an increased mortality risk, with an odds ratio (95% confidence interval (CI)) of 27.8 (6.3–122.9) (Shoar et al, 2020) and a reported mortality rate of approximately 50% (Chen et al, 2020; Dan et al, 2020). A number of underlying factors and common comorbidities may compound the person's risk of poor outcomes of heart failure with COVID-19. The interplay between respiratory and cardiovascular homeostasis is apparent, but underlying biochemical dysfunction in heart failure may also contribute to the pathogenesis of COVID-19 infection. Patients with heart failure have an elevated and disorganised inflammatory response, that, in itself, is mutifactorial and relates to obesity, ischaemic changes and cachexia (Rocha and Libby, 2009; Rahman et al, 2016). Heart failure and COVID-19 affect many of the same body systems, including the renal, respiratory, haematological and gastrointestinal systems (Gavriatopoulou et al, 2020). This may further destabilise the involved systems and cause further systemic dysfunction.
Physiological mechanisms that that are directly linked to COVID-19 include angiotensin converting enzyme 2 (ACE-2). ACE-2 has gained recognition as the receptor by which SARS-CoV-2 enters cells (Razeghian-Jahromi et al, 2020). Therefore, the location and number of ACE-2 receptors will accordingly help determine access of SARS-CoV-2 to tissues and organs. Moreover, pathological or pharmacological influences on ACE-2 may affect the receptor, consequently influencing the progress of COVID-19 infection. To help understand this pivotal aspect of COVID-19 pathogenesis, a brief synopsis of ACE-2 physiology is presented below.
Similar to the ACE-1 enzyme, ACE-2 catalyses the breakdown of angiotensin-1, which produces angiotensin II (Ang II). Degradation of Ang II by ACE-2 produces angiotensins 1-7 (Ang 1-7). Via the Mas receptor, Ang 1-7 produces an array of effects that counteract the main effects of Ang II (Figure 1). These effects of ANG 1-7 are cardiovascular protective and include anti-ischemic, antithrombotic and anti-proliferative effects (Santos et al, 2018). Together, these effects reduce ischaemic and hypertrophic influences on the heart and blood vessels. The Mas receptor is expressed in a number of different organs in the body, including the blood vessels of the heart, kidneys and lungs (Santos et al, 2018). In these locations, Ang 1-7 mediates an effect that is typically opposite to that of Ang II; notably, Ang 1-7 is largely vasodilatory at physiological concentrations as well as anti-proliferatory and anti-inflammatory, particularly in the lungs. The binding of the SARS-CoV-2 virus via its spike protein (Figure 2) to the ACE-2 receptor leads to internalisation of the virus receptor complex, which results in a reduction in the number of ACE-2 receptors on the cell surface (receptor down-regulation) (Mahmudpour et al, 2020). Consequently, the beneficial inhibitory effects of the ACE-2 receptor are lost, allowing the effects of Ang II to be magnified.
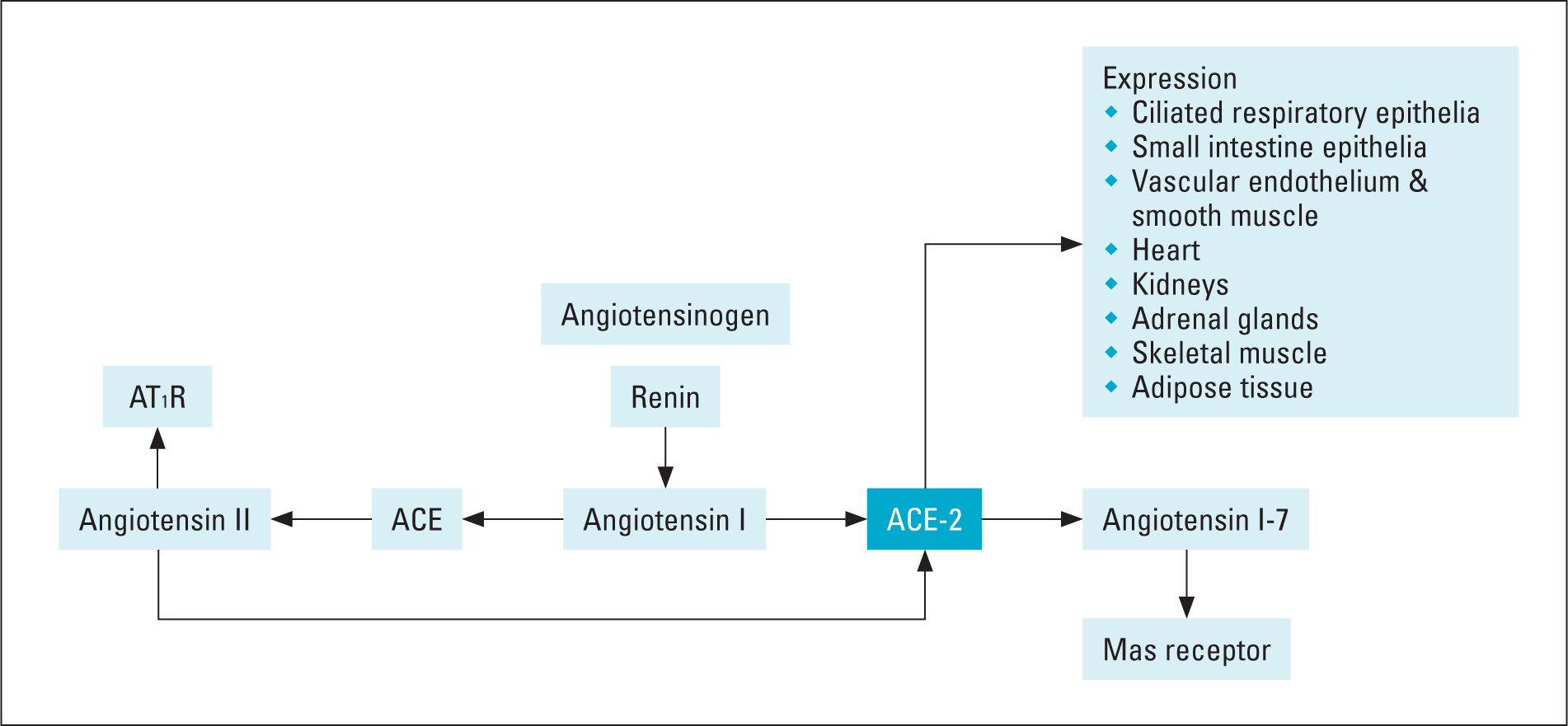 Figure 1. Physiology of ACE-2 receptor and its location
Figure 1. Physiology of ACE-2 receptor and its location 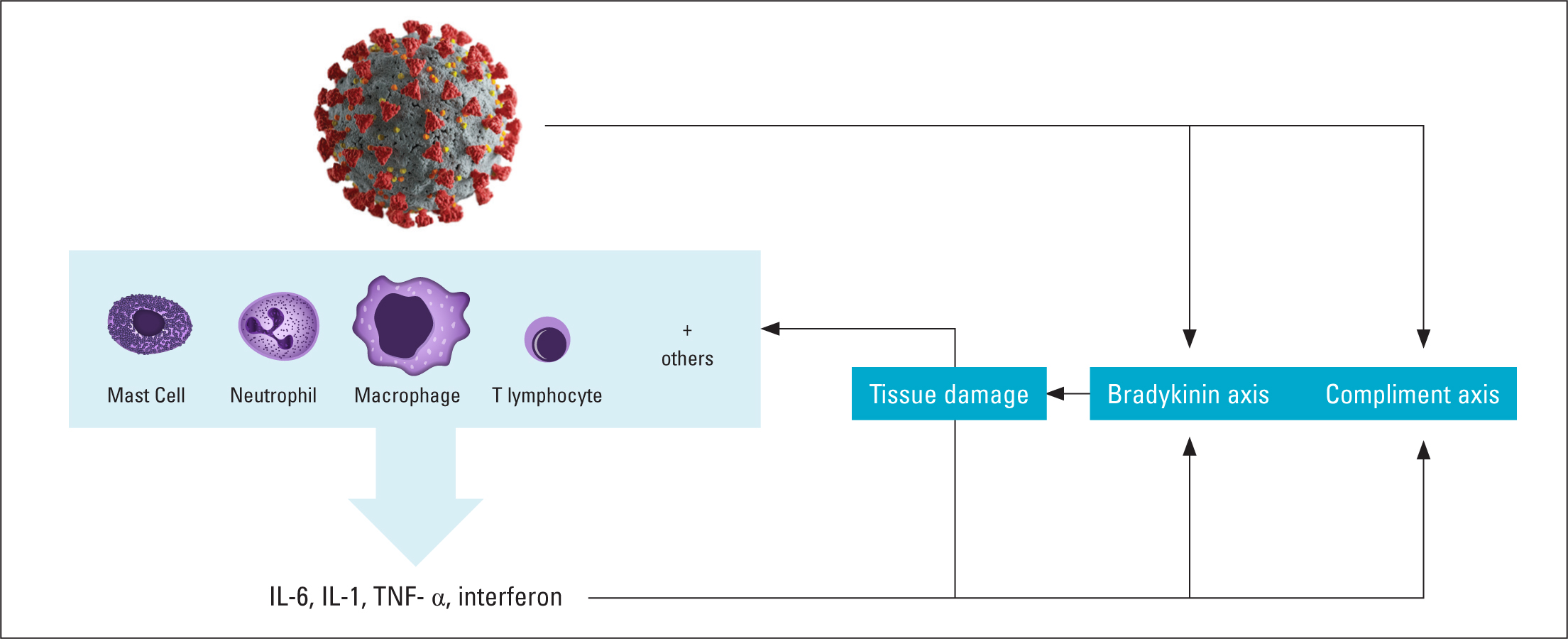 Figure 2. Overview of inflammatory response in COVID-19
Figure 2. Overview of inflammatory response in COVID-19
ACE-2 receptor expression
Given the importance of the ACE-2 receptor in COVID-19 pathogenesis, an understanding of the expression of the ACE-2 receptor helps explain some of the effects of COVID-19. The ACE-2 receptor is widely expressed in a number of organs. Consequently, as SARS-CoV-2 uses this receptor to enters cells, these organs are particularly susceptible to a direct attack from the virus. The main area of expression of the ACE-2 receptor is the differentiated (specialised) epithelium, which is mainly found in abundance in the lungs and small intestines. Other organs that express the ACE-2 receptor, but to a lower extent, are the vascular endothelium, smooth muscle, heart, kidneys, adrenal glands, skeletal muscle and adipose tissue (Santos et al, 2018) (Figure 3). It is not surprising, therefore, that the main symptoms of COVID-19 include the respiratory system, gastrointestinal tract and musculature (Esakandari et al, 2020).
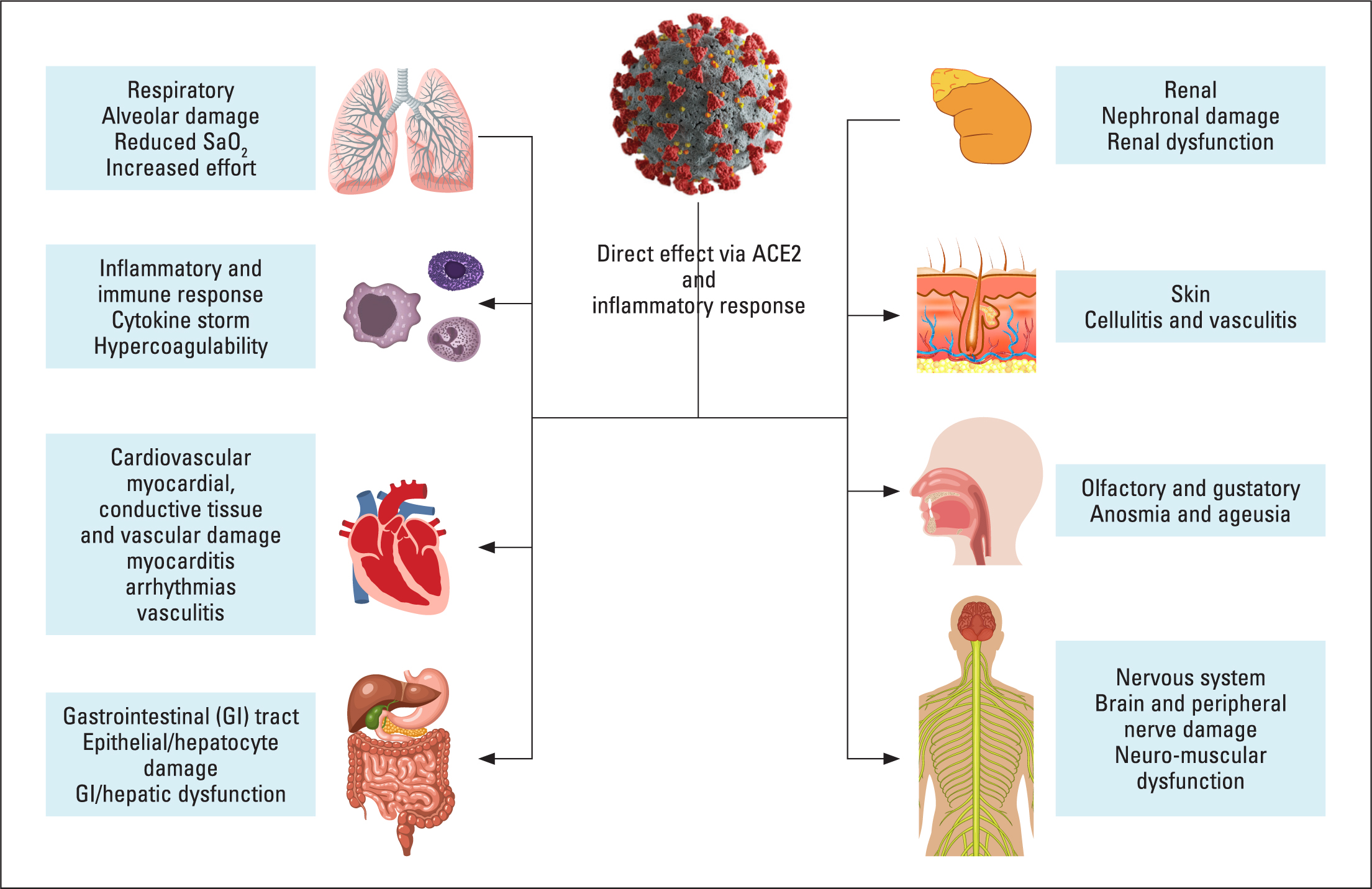 Figure 3. Main effects of COVID-19 on the body
Figure 3. Main effects of COVID-19 on the body
COVID-19 has many significant and detrimental effects on patients with heart failure. Understanding the main manner in which COVID-19 affects patients with heart failure may help with the monitoring and management of these patients.
Cardio-respiratory effects of COVID-19 in patients with heart failure
Patients with heart failure present a number of cardio-respiratory challenges. Insufficient cardiac function in heart failure activates, among others, the sympathetic nervous system, which results in an increase in the heart rate and cardiac workload, a process that is well known to exert the already failing heart (Porzionato et al, 2020). The increased sympathetic nerve tone is further compounded by the presence of hypoxia in COVID-19 patients. Together, these two stimuli produce a number of adverse effects, such as increased cardiac workload and the risk of arrhythmias.
An increased cardiac workload will accelerate deterioration of cardiac dysfunction. One of the main symptoms of worsening cardiac function in heart failure is pulmonary oedema. The presence of pulmonary inflammation, of any form, will have a detrimental effect on pulmonary oedema, as pulmonary inflammation leads to loosening of the junctions between alveolar cells, leading to alveolar oedema (Wittekindt, 2017). For this reason, COVID-19-related alveolar inflammation will aggravate heart failure-related pulmonary oedema already present, worsening gaseous exchange and patient outcomes.
A spectrum of arrhythmias have been reported in COVID-19 patients (Babapoor-Farrokhran et al, 2020b), including tachycardic and bradycardiac arrhythmias. However, three main factors predispose patients with heart failure to tachycardiac arrhythmias, increased sympathetic nerve tone, dilated and fibrosed myocardium and presence of polypharmacy, increasing the risk of QT prolongation. COVID-19 may detrimentally influence all these factors by attacking the myocardium directly, as well as its effect via sympathetic nerve tone. Pharmacological treatments used to treat COVID-19 patients, including chloroquine and hydroxychloroquine, are known to prolong the QT interval (Bader et al, 2020). Antibiotic and antiviral combinations used to treat COVID-19 patients may further contribute to QT prolongation. Heart failure-induced renal dysfunction may also affect drug clearance, which increases the risk of drug interactions, including QT prolongation.
Immune and thrombotic response associated with COVID-19 in patients with heart failure
Heart failure is understood to be associated with a chronic low-grade inflammatory response (Vulesevic et al, 2019). Given their elevated inflammatory baseline, patients with heart failure may influence and may be influenced by other inflammatory events. COVID-19 patients are at risk of experiencing a severe acute inflammatory response, the so-called ‘cytokine storm’ (Mahmudpour et al, 2020). Several factors appear to contribute to cytokine storms in COVID-19 patients, some of which have direct effects, including the general inflammatory response together with the associated activation of the complement cascade. Other indirect but equally important factors include involvement of the ACE-2 receptor, an elevated sympathetic nerve tone and probably obesity (Magdy Beshbishy et al, 2020). Similarly heart failure-related cachexia, which is associated with a reduced availability of nutrients and is an inflammatory process in its own right (Rahman et al, 2016), may contribute to the inflammatory milieu.
The inflammatory process is complex and includes many cellular responses and release of inflammatory mediators, which stimulate or amplify the response (cytokines). Entry of SARS-CoV-2 into the body triggers the innate immune response. This initial response is mediated by several different lymphocytes and white blood cells (Figure 2). These cells, in turn, secrete an array of cytokines, of which interleukin (IL)-6 and IL-1, tumour necrosis factor-alpha (TNF-α) and interferon are of particular importance (Ragab et al, 2020). Presence of these cytokines results in a significant inflammatory response leading to further recruitment of inflammatory cells into the area of infection, resulting in barrier membrane breakdown, oedema formation and cell death. These mechanisms lead to localised and systemic organ dysfunction, which may lead to organ failure and death. The presence of TNF-α is significant in patients with heart failure. TNF-α was formally known as cachexin, a cytokine found to be elevated in cachectic heart failure patients (Rahman et al, 2016). Elevated TNF-α levels are a sign of severe illness in COVID-19 patients (Li et al, 2020; Pedersen and Ho, 2020).
The prevalence of an overt cytokine response or cytokine storm appears to stem from poor anti-inflammatory responses, together with other co-factors that promote inflammation. It has been suggested (Mahmudpour et al, 2020) that COVID-19-mediated downregulation of the ACE-2 anti-inflammatory response may contribute towards an amplified inflammatory response seen in some COVID-19 patients. Downregulation of ACE-2 would result in an unchecked ACE1-angiotensin II response that is pro-inflammatory, together with a reduction in ACE-2-mediated breakdown of inflammatory components of the bradykinin pathway. Similarly Ang II may further aggravate inflammation by activating components of the complement cascade (Laecke and Biesen, 2017). Factors that may further complicate the inflammatory response include obesity, a known risk factor for COVID-19 morbidity, which may disrupt inflammatory regulation (Magdy Beshbishy et al, 2020). Similarly a heightened sympathetic nervous system response linked to Ang II and hypoxia may stimulate inflammation (Porzionato et al, 2020).
In addition to tissue damage and organ dysfunction, the inflammatory response initiates and potentiates thrombosis. Coagulopathies are a common feature of COVID-19 infection and correlate with inflammation, morbidity and mortality (Levi et al, 2020; Li et al, 2020). Of the clotting markers examined, a modest prolongation in prothrombin time and modest reduction in platelet count, together with elevated levels of D-dimer, were common (Connors and Levy, 2020; Levi et al, 2020). Elevated D-dimer levels are of significance, as they are correlated with mortality (Connors and Levy, 2020; Yu et al, 2020). It is important to note that elevated D-dimer levels are also a predictor for all-cause mortality in heart failure (Huang et al, 2019; Yan et al, 2019; Zorlu et al, 2012). These two findings reinforce its importance as a monitoring marker in patients with heart failure and diagnosed or suspected COVID-19 infection. Interestingly, current understanding suggests that elevated D-dimer levels found in COVID-19 patients are correlated more strongly with severity of inflammation and disease rather than coagulation state, as only modest increases are observed in the levels of other coagulation markers (Connors and Levy, 2020).
Both COVID-19 and heart failure are recognised as having significant inflammatory components to their pathogenesis, the presence of which significantly contributes towards the prognosis of both conditions independently. Combination of these two inflammatory comorbidities will have significant consequences for the patient. Therefore, these comorbidities present a synergistic mix of pathologies and responses that lead to a complicated and variable clinical presentation. These interactions result in an elevated sympathetic nerve tone that further increases a heightened Ang II response (Figure 4), and together, these stimulate an already elevated inflammatory response. Environmental factors, such as weather and nutrition, may further impact both conditions by increasing the risk of secondary bacterial infection and poor nutrient availability, which is vital to maintain an adequate immunological response.
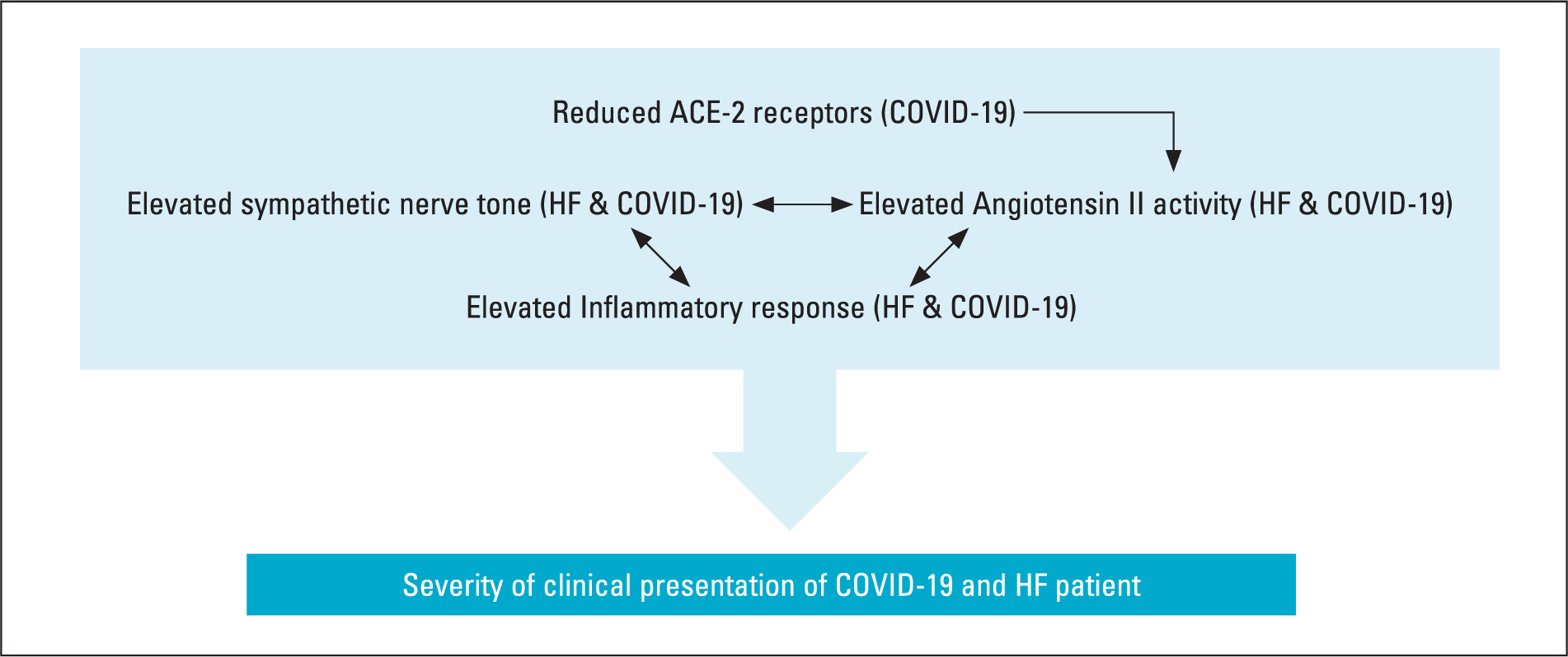 Figure 4. Interaction between key elements that affect heart failure and COVID-19, leading to increased morbidity
Figure 4. Interaction between key elements that affect heart failure and COVID-19, leading to increased morbidity
The overall outcome of these interactions is:
- An increased risk of a cytokine storm, with consequent sequelae of organ dysfunction/failure and coagulopathies
- Increased cardiac workload, leading to poor haemodynamic function and increased risk of arrhythmia
- A general catabolic state that will increase cachexia and reduce the immune response.
Implications for practice
It has been reported that only a few patients with acute heart failure admitted to hospital during the first wave of COVID-19 infection (Bromage et al, 2020; Cannata et al, 2020), although the patients who were admitted had a comparative higher NYHA score (III-IV) than previous years and experienced a higher mortality rate. Given the epidemiology and pathogenesis of both pathologies, acute care of the patient with COVID-19 and heart failure will probably require hospitalisation. However, primary care has a role to play, both in relation to prophylaxis as well as post-hospital discharge care, together with caring for patients with heart failure and COVID-19 who are managed in the community during the acute phase of their infection.
There are no clear pathways of care related to COVID-19, and treatment remains empirical and related to symptom management (Tajbakhsh et al, 2020). Risk factors for poor outcomes of COVID-19 infection include:
- Male gender
- Older age
- Elevated lactate dehydrogenase levels
- Raised D-dimer levels
- Leukocytosis
- Hyperglycaemia
- Raised troponin levels.
Much of what is known of COVID-19 mortality risk factors has come from Chinese populations (Chen et al, 2020; Li et al, 2020) that may possess particular polymorphisms not shared by the global population. However, the identified factors appear to be commensurate of what is understood of the pathogenesis of the disease, suggesting that these mortality risk factors may be globally applicable. Care for patients with heart failure in relation to COVID-19 should then address issues related to the abovementioned risk factors.
How well these issues translate into primary care in the UK is debatable. Markers such as lactate dehydrogenase and D-dimer are not commonly monitored in the community and, given their association with disease severity, it is likely that the person may already be admitted before the marker's level becomes relevant. As with all clinical decisions, one turns to clinical guidelines for assistance. However, although there are clinical guidelines for COVID-19-infected patients with acute myocardial damage (National Institute for Health and Care Excellence (NICE), 2020), there are no specific guidelines for patients with heart failure and COVID-19 as yet, either as in-patients or for patients managed in the community.
Based on the analysis of the pathogenicity of both COVID-19 and heart failure, some basic conclusions may be drawn. Given the effect that both conditions have on coagulation and its link to disease severity, this is an area of importance. Atrial fibrillation (AF) is, as with common forms of heart failure, a disease of the older person, and, for this reason, both these conditions frequently co-exist (Shah et al, 2018). It has already been established that heart failure and COVID-19 increase coagulation risk; adding AF to this mix may further worsen the risk, although this requires verification (Gawałko et al, 2020). For patients with AF receiving treatment with anticoagulants, it is possible that the coagulogenic effect of AF may be somewhat mitigated. However, in patients with heart failure with new-onset AF triggered by COVID-19 (Babapoor-Farrokhran et al, 2020b) or asymptomatic AF, so-called silent or sub-clinical AF (Dilaveris and Kennedy, 2017; Staerk et al, 2017), sufficient anti-coagulation regimens may not be in place.
Early detection of symptomatic or silent AF will, therefore, help timely initiation of anti-coagulation therapy. Self-monitoring using palpable pulse irregularity as well as rhythm strip analysis remains the mainstay strategy to monitor for AF in the community (Kirchhof et al, 2016). However, the available new technologies do demonstrate good sensitivity and specificity when diagnosing AF (Dilaveris and Kennedy, 2017).
The risk of drug-induced arrhythmias may also be present in patients with heart failure and COVID-19. Drug-induced arrhythmias may relate to tachycardic and bradycardic episodes depending on the medication used. However, the risk of QT prolongation is a significant concern. It has been advised that, if a patient's QT interval corrected for heart rate (QTc) exceeds 500 msec, then the culprit medication should be terminated (Trinkley et al, 2013) as a QTC > 500 msec significantly increases the risk of torsades de pointes (TdP). Patients with heart failure and COVID-19 possess a significant risk of QTc prolongation. This increases the risk of TdP or polymorphic ventricular tachycardia. These factors include:
- Underlying heart disease related particularly to cardiomyoparthy and fibrosed muscle
- Polypharmacy, increasing the risk of drug interaction and consequent QT prolongation
- Unstable renal function that may effect drug clearance
- Unstable electrolyte levels, particularly reduced serum potassium and magnesium levels
- Increased sympathetic nerve tone
- Increased possibility of taking medications that affect the QT interval (e.g. antiarrhythmics, some antibiotics and antiviral medications).
The risk of QT prolongation is not easy to assess (Tomaselli Muensterman and Tisdale, 2018). The Tisdale score is one such assessment; it has only been validated in the hospital setting (Tisdale et al, 2013), but has been used to assess QTc prolongation risk in drug combinations used in treating COVID-19 patients in hospital (Bianco et al, 2020). A systematic review of tools that predict QT prolongation risk (Tomaselli Muensterman and Tisdale, 2018) has demonstrated that assessments including genetic factors provide best predictive value. However, the availability of this information in primary care practice will be variable. Studies conducted using drug combinations commonly used in COVID-19 patients have provided some insight into QT prolongation risk in this patient group.
A systematic review of drug combinations (hydroxychroloquine (HCQ) and azithromycin (AZ)) reported to increase QTc (Agstam et al, 2020) found a drug-induced increase in QTc of >500 msec in 20 of a cohort of 196 COVID-19 patients, all of whom had a high Tisadale score (≥11). However, no instances of TdP were reported. Although patients with coronary artery disease were identified in this review, the presence of heart failure was not reported. In another study (Ramireddy et al, 2020), of the 98 eligible participants, 27% received AZ, 10% received HCQ and 61% patients received both medications. The combination therapy demonstrated a more pronounced prolongation of QTc compared with the single medications. Only 20% of the population had heart failure, but despite QTc prolongation, including 12 patients with a prolonged QTc >500 msec, no participant experienced TdP. Therefore, the actual risk of QTc-mediated TdP in patients with heart failure and COVID-19 is unclear, this may be because of a lack of studies examining this particular mix of comorbidities. Given the proven link between QTc prolongation and TdP and evidence that drug combinations used in COVID-19 and heart failure cause significant QTc prolongation (> 500 msec), vigilance in monitoring QTc in patients with heart failure and COVID-19 is warranted. Further, since pharmacokinetic drug interactions can influence QTc prolongation, medication reviews and, where needed, deprescribing, should form part of the QTc monitoring process.
Management of patients with heart failure and COVID-19 is empirical and depends on symptoms presented. A catabolic state is a common feature of both disease processes. Therefore, nutrition is of significant importance in both conditions. Overall dietary considerations in COVID-19 patients, as with patients with heart failure, are focused on an anti-inflammatory diet that has a low glycemic index and is high in omega 3 fatty acids (Iddir et al, 2020). High-biological value protein-such as fish, eggs, lean meat such as chicken, and whey protein-is important in a low inflammatory diet and fortifies the immune response (Iddir et al, 2020). Replacing animal protein with polyphenol-rich fruit and vegetables, such apples, olives and olive oil, together with turmeric, may also be beneficial (Cena and Chieppa, 2020).
Vitamin D deficiency has been identified as a factor linked to COVID-19. This assumption has been based on the importance of vitamin D in the immune response (Bleizgys, 2020). To date, a conclusive link between vitamin D deficiency and COVID-19 infection and outcomes is absent (Bleizgys, 2020; Pizzini et al, 2020). However, studies have shown that vitamin D deficiency does correlate with an increased risk of poor outcomes with invasive mechanical ventilation and death in those with COVID-19 (hazards ratio (HR)=6.12, 95% confidence interval (CI)=2.79–13.42, p < 0.001; HR=14.73, 95% CI=4.16–52.19, p < 0.001, respectively) (Radujkovic et al, 2020). Vitamin D also plays a role in heart failure, where it is reported to provide a TNF-α-related anti-inflammatory effect (Rodriguez et al, 2018), as well as improving ventricular dimension remodelling and ejection fraction (Zhao et al, 2018). Minimal serum vitamin D levels have been debated, but >30 ng/ml serum 25-hydroxy Vit D (a marker for serum vitamin D levels) is recommended (Bleizgys, 2020; Pizzini et al, 2020; Radujkovic et al, 2020). Although a higher level for at risk groups (40–60 ng/ml) has been suggested, translating to a 2000–5000 IU daily dose for at-risk groups (Bleizgys, 2020). Other micronutrients that improve immune response include vitamins A and C and zinc (Hannan et al, 2020; Iddir et al, 2020).
Conclusion
Patients with heart failure are significantly susceptible to COVID-19 infection. The combination of heart failure and COVID-19 in an individual leads to a synergistic increase in the core disease process of inflammation and sympathetic nerve pathway activation. These effects result in an increased risk of a cytokine storm, coagulopathies, arrhythmia and a catabolic environment. Together, these processes increase morbidity and mortality. Understanding these circumstances enhances clinical monitoring and directs appropriate care.
KEY POINTS
- Heart failure and COVID-19 are pro-inflammatory conditions that promote the risk of a cytokine storm, with the resulting risks of organ dysfunction, coagulopathies and a catabolic state
- The combined co-morbidities may precipitate arrhythmias, resulting in atrial fibrillation and ventricular tachyarrhythmias. To prevent this, electrolyte balance needs to be maintained and strategies to regularly monitor for AF need to be adopted
- Polypharmacy resulting from the increased drug burden related to COVID-19 and heart failure increase the risk of QTc prolongation, which needs to be monitored
- The combined catabolic and inflammatory state experienced by people with these comorbidities requires that diet is prioritised, with a focus on providing an anti-inflammatory diet that fortifies the immune response
CPD REFLECTIVE QUESTIONS
- How might a combination of heart failure and COVID-19 increase the risk of stroke?
- Why is monitoring for atrial fibrillation in a patient with heart failure and COVID-19 important?
- What kinds of diets may promote heath and recovery in patients with heart failure and COVID-19 and why?
- What is the importance of medication review in patients with heart failure and COVID-19?


