The global search for a solution to the COVID-19 pandemic is intensifying, as countries are attempting to return to normalcy after lockdown. In a series of articles, the author aims to revisit clinical knowledge of how viruses cause human infections to facilitate comprehension of additional material exploring the challenges and potential wins in the battle against this devastating pandemic.
How do viruses infect human beings?
To cause disease in an animal, including humans, viruses require host specificity, which dictates the reservoir and host susceptibility to invasion. Influenza and coronaviruses enter the human body mainly via droplet transmission through exposure of mucous membranes that connect with the respiratory system (Kutter et al, 2018; Wilson et al, 2020). Aerosols would travel greater distances, however, dilution while suspended in air and surface contact render these a transmission risk for face-to-face contact within 2 m in most human-to-human interactions (World Health Organization (WHO) 2014; Bourouiba, 2020).
On contact with a mucous membrane, the virus particle must be able to attach to and infiltrate the host cell. Using inherent proteins, the virus dissembles its parts and replicates the essential information in genetic code to engineer mini-versions of itself, before releasing virions that bud from the host cell (Lim et al, 2016). This process depends on compatible molecular interaction between the host cell and, in the case of the enveloped coronavirus responsible for COVID-19, the viral membrane (Du et al, 2020; Hussain et al, 2020).
How does SARS-CoV-2 spread in human beings?
Severe acute respiratory syndrome coronavirus (SARS-CoV-2) is the name given to the virus responsible for the disease COVID-19 that it causes in humans (World Health Organization (WHO), 2020a).
Like flu viruses and other human coronaviruses (Corman et al, 2018), SARS-CoV-2 is spread primarily through droplets (respiratory secretions) and close person-to-person contact. Smaller aerosols of <5 microns could be generated from medical interventions involving the paranasal mucosa and respiratory epithelial cells. Viruses travel greater distances while suspended in air, and indoor settings with poor ventilation may present a higher risk, particularly during clinical aerosol-generating procedures if conducted on an infected patient (Morawski, 2020; WHO, 2020b). Conversely, well-ventilated and outdoor spaces rapidly dilute aerosolised viral particles expelled from the respiratory tract, rendering face-to-face contact in these settings less likely to lead to transmission in susceptible hosts.
Environmental contamination could occur via indirect contact transmission routes. For example, viral particles from an infected reservoir/host are expelled via coughing, sneezing and, as implicated by one study, shouting (Asadi et al, 2019), and then get dispersed into the immediate surroundings and onto inanimate surfaces. When a cough, sneeze or respiratory mucosal secretion is caught in the hands, and these hands subsequently touch surfaces such as door handles, keys (referred to as fomites) or other people, viral matter is inevitably transferred (Chia et al, 2019; Akram, 2020).
The normal flora of the skin is unique to each individual (Sohn et al, 2018), and this microbiome, although not set, functions as a protective first line of defence against opportunistic bacteria and viruses. Transient colonisation of the skin around and below the upper respiratory tract with virus shed from the mucous membranes during the early period of viral replication is a likely method by which the virus spreads from an infected individual who inadvertently touches their face, nose, mouth or eyes and leaves this genetic matter as a contaminant on the surfaces they touch (Bosch et al, 2013).
For this reason, hand hygiene-washing hands with water and soap, following a proscribed technique (WHO, 2009)-is critical in the prevention of cross-infection and spread of infectious disease. Alcohol-based sanitisers (minimum 62–71% ethanol equivalent) are effective against influenza and coronaviruses and are recommended if the hands are socially clean, that is, not visibly soiled (NICE, 2014; WHO, 2020c).
Wearing gloves without an evidence-based rationale risks inappropriate use. Ill-considered glove-wearing increases user risk of skin sensitivity to proteins used in the manufacture of gloves and is ecologically difficult to justify. Recent occupational hazard awareness and environmental concerns have been the focus of recent professional campaigns to reduce glove-wearing where not clinically indicated (Royal College of Nursing, 2020). A less tangible environmental impact of SARS-CoV-2 may well be the dilution of informed professional policy by nebulous public information during the pandemic. Nitrile, vinyl and latex gloves provide a warm, moist environment in which the transient and resident flora on the hands of the wearer will be encouraged to multiply, further increasing the risk of local skin damage and dermatitis. Gloves are not a substitute for hand hygiene; hands should still be decontaminated with the same frequency whether gloves are worn or not, as the level of contamination on the gloves will be equivalent to whatever the gloved hand has touched. Gloves are not indicated unless significant contamination is anticipated with a hazardous substance, including an infectious agent from another person, for example, when handling body fluids and respiratory secretions (European Centre for Disease Prevention and Control, 2020).
Fomites and environmental fabric have been implicated as factors in nosocomial outbreaks and sustained transmission of viral illness. Mathematical and interventional studies have indicated that self-inoculation of the eyes, mouth and nose (mucous membranes) by healthcare workers' hands contaminated by the environment is an important focus for infection control interventions (Otter et al, 2016). Applying the same theory of cross-contamination, used tissues or handkerchiefs may effectively provide a short-term reservoir for viral particles, which, when safely ensconced in physical matter, for example, skin cells, mucous proteins and moisture, are protected from drying out and may survive as a viable infective agent for longer periods. Reuse of these items contaminates hands and clothing and, in a more hospitable temperate medium close to the warmth of the human body, is likely to potentiate survival. Viable viruses and SARS-CoV-2 RNA have been identified shed or dispersed onto impermeable, dry surfaces (Dowell et al, 2004; WHO, 2020b). In one study, 56.7% of the 30 airborne-isolation rooms had at least one environmental surface contaminated despite 12 air changes per hour (Chia et al, 2020).
To control the environmental role in transmission, it is evidenced that influenza and coronaviruses are easily damaged by detergents and killed by 0.1% sodium chloride (Akram, 2020) or, in clinical terms, 1000 ppm available chlorine-based products. A documented and specified cleaning process is required to ensure the appropriate frequency of cleaning-especially of high-contact touch points-and the right concentration of product to deliver environmental decontamination or destruction of viable viral matter.
To reduce the risk of environmental contamination, it is good practice to seal contaminated soft waste in a small bin liner at source; sharp waste requires safe disposal in a rigid container at the point of use (Health and Safety Executive (HSE), 2013). In practice, this means the required double bagging of COVID-19-contaminated waste happens when placed in the larger waste receptacle. In a patient's home, this would be the domestic waste stream and, within a healthcare facility, a colour-coded bin liner that denotes the hazard category: hazardous, offensive or domestic. The virus does not survive long as a viable pathogen outside of human cells and coronavirus-contaminated waste is therefore rendered safe for domestic waste collection in the community after 72 hours.
How does SARS-CoV-2 infect humans?
Appreciating how the virus breaches host defences underpins many of the proof of concept workstreams on vaccine research to date; the aim is to minimise the likelihood of an exposed individual developing disease. The detail of this process is important to ascertain possible sites for targeting interventions designed to optimise cell-mediated immunity or neutralising-antibody production (Alharbi et al, 2017).
After the virus connects with the mucosal surface of the human host, it binds to epithelial target cells that express virus receptor angiotensin-converting enzyme 2 (ACE2).
The coronavirus is so called on the basis of the crown or halo-like appearance given by the glycoprotein-studded, spiked envelope on electron microscopy (Tyrell and Myint, 1996) (Figure 1). The spikes of SARS-CoV are composed of trimers of S protein, which belongs to a group of class I viral fusion glycoproteins that, interestingly, also includes the influenza haemagglutinin protein. The SARS-CoV spike (S) protein is composed of two subunits: the S1 subunit contains a receptor-binding domain that engages with the host cell receptor ACE2, and the S2 subunit mediates fusion between the viral and host cell membranes (Du et al, 2020). The presence within the spike protein of an amino acid site (polybasic site) allows its functional processing by the human enzyme furin (protease), leading to the fusion of the viral and cell membranes, a necessary passage for the virus to infiltrate the cell (Cascella et al, 2020).
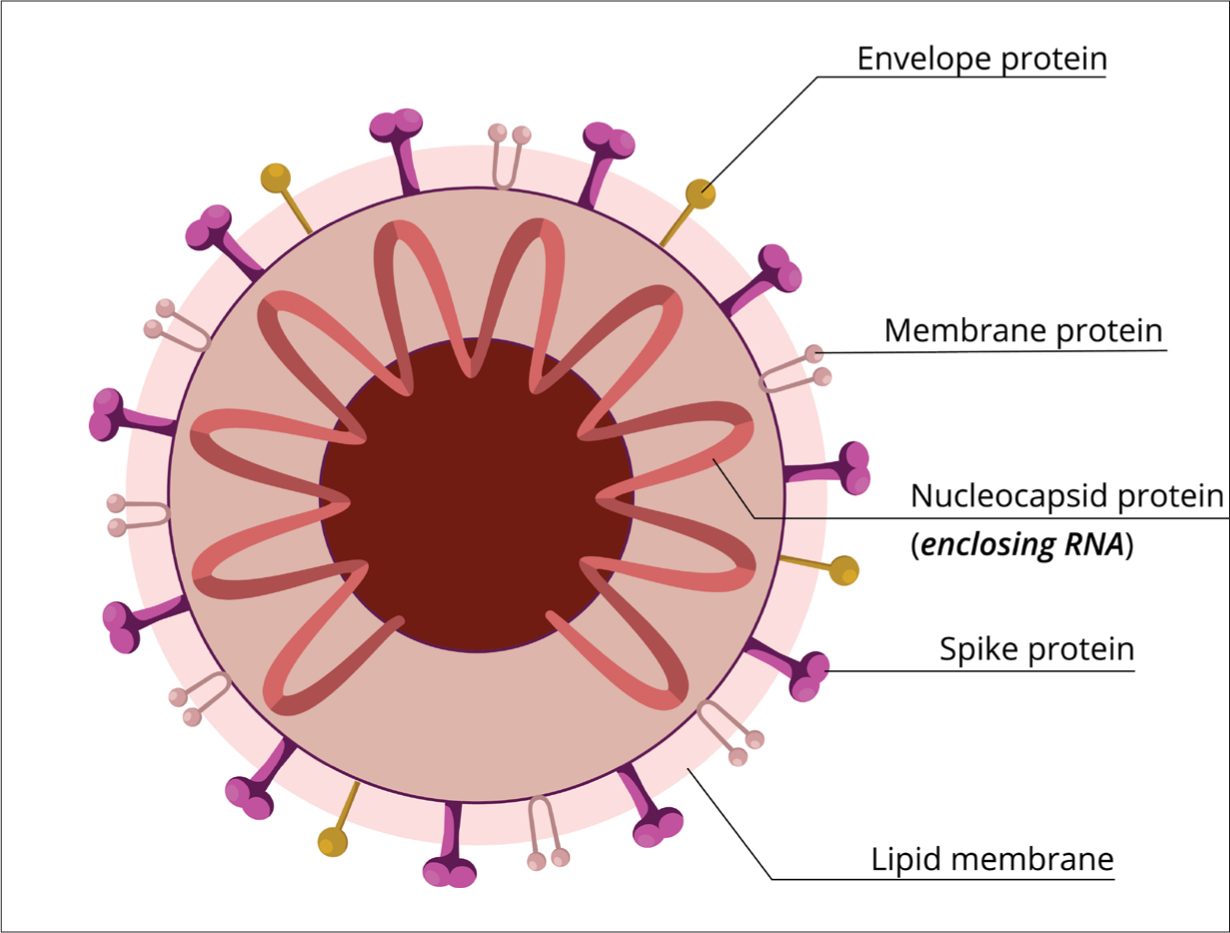 Figure 1. Structure of coronaviruses
Figure 1. Structure of coronaviruses
Replication of the virus from within the host cell is specific to the species of virus, and the viral genome for SARS-CoV-2 is carried on the molecules of the nucleic acid. Its single-stranded RNA genome contains 29 891 nucleotides, encoding 9860 amino acids (Chan et al, 2020). Within the cell, the virus loses its membrane and sheds the capsid to allow the synthesis of the viral RNA releasing viral nucleic acid. Starting from the viral RNA, the synthesis of polyproteins begins. In the atypical CoV genome, at least six open reading frames (ORFs) work as templates for the replication-transcription production of subgenomic messenger RNAs; the SARS-CoV-2 genome has 12 ORFs (Chan et al, 2020). ORFs encode for structural proteins, including the spike, membrane, envelope, and nucleocapsid proteins, and accessory proteic chains (Perlman et al, 2009).
Using the relocated nucleic acid, the virus particles form a new nucleocapside within the host cell. The final element of replication occurs as the envelope proteins and glycoproteins, translated from mRNA bud to and acquire the host cell membrane. At this point, the altered antigenic components of the expressed membrane function to inform the cell-mediated immune response pathway (Goering et al, 2010).
The matured virions are released to infect new target cells. SARS-CoV can infect the intestines via enterocyte infiltration, tubular epithelial cells of the kidneys, epithelial cells of the renal tubules, cerebral neurons and immune cells. Presence of SARS-COV-2 RNA has been identified in faeces and urine, although there is little evidence this constitutes a route of transmission (WHO, 2020c).
SARS-CoV infection damages lung tissues owing to elevated levels of production and activation of pro-inflammatory chemokines and cytokines, resulting in atypical pneumonia with rapid respiratory deterioration and failure (Du et al, 2009). The hypotheses that the spike protein and host receptor play key parts in the induction of neutralising antibody and T-cell responses, as well as protective immunity during infection with SARS-CoV, make these prime targets for vaccine and therapeutic development. By inhibiting the binding mechanism that reduces the replication and subsequent viral load in the lungs, and thereby limiting pulmonary damage, more severe disease can be prevented at the individual patient level (Belen-Apak and Sarialioglu, 2020).
Hussain et al (2020) referred to the variation in clinical manifestations and recovery rate of COVID-19 among different age groups, nationalities and races. They suggested not only that natural genetic variants in host receptors may offer susceptibility or resistance against evolving pathogenic viruses, but also that the existence of ACE2 variants may indicate a protective element. It was inferred that the binding site of the coronavirus spike and the glycoprotein alleles enabling or inhibiting connection may explain why some people are more susceptible to infection and experience poorer outcomes than others.
Predictors of poor outcomes
Cardiovascular disease, obesity and advanced age are predictors of poorer outcome of COVID-19. Bannerjee et al (2020) developed a tool by which individual risk can be predicted across underlying and predisposing conditions. The benefits to clinical, public health and research decision-makers of a modelled risk profile for both the COVID-19 pandemic and post-pandemic contexts can be realised in therapeutics as researchers seek to prevent the most vulnerable in society from the poorest outcomes. Such a risk profile also provides a strong clinical, ethical and financial evidence base for who should become the priority target groups for vaccination now that a SARS-CoV-2 vaccine has become an accessible reality.
Conclusion
As this article is being written, the second wave of coronavirus cases is well underway, and there is an increasing sense of urgency that the global community coordinate the learning around this disease. This will ensure that the science of robust infection prevention and control underpins practice and behaviours to enable health care and economic systems to continue to function through this next critical period.
Current priority research areas for the WHO (2020d) focus on a greater understanding of the natural history of the virus, including its zoonotic ability to transfer between species, and opportunities for therapeutic interventions to minimise both the rate of infection and the individual experience of system damage caused by COVID-19.
The related articles in this series introduce and explore these concepts to support clinical awareness and practice guidance.
KEY POINTS
- The second wave of the COVID-19 pandemic has begun in the UK, and various measures are being implemented to curtail its effects
- To combat the COVID-19 pandemic, it is important to understand how SARS-CoV-2 spreads and infects human beings
- A current research focus is obtaining a better understanding of the natural history of the virus
- There are several predictors of poor outcomes of COVID-19 infection, including diabetes, high BMI and advanced age
CPD REFLECTIVE QUESTIONS
- What is the process by which SARS-CoV-2 infects human beings?
- What are the factors that decide the outcomes of SARS-CoV-2 infection?
- How would having a better understanding of the science underlying the COVID-19 pandemic and the virus responsible help combat the pandemic?


